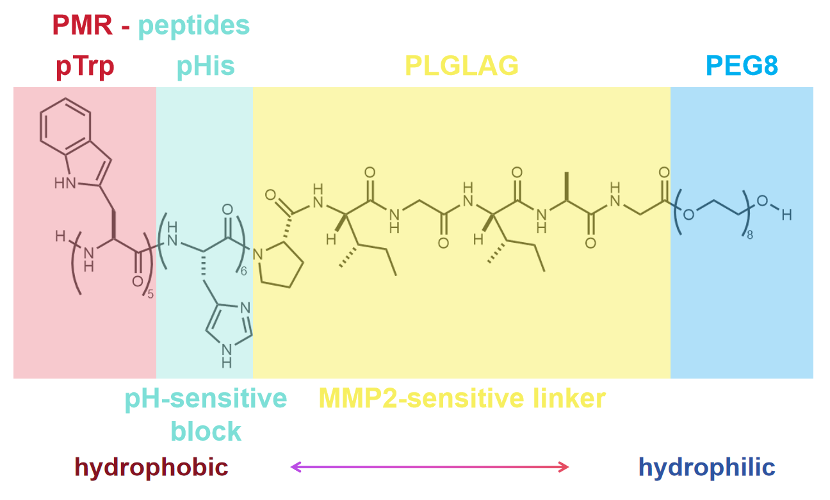
Generalized
Here we report a self-assembled nano antitumor peptide with the unique capability to trigger cell death across a wide spectrum of cancer cell phenotypes through plasma membrane rupture (PMR) upon stimuli from tumor microenvironment, such as pH and the overexpressed matrix metalloproteinase 2 (MMP-2). The designed peptide consists of a hydrophobic poly(tryptophan)-poly(histidine) (pTrp-pHis) core conjugated with a hydrophilic polyethylene glycol (PEG-8) chain through a peptide linker (PLGLAG).
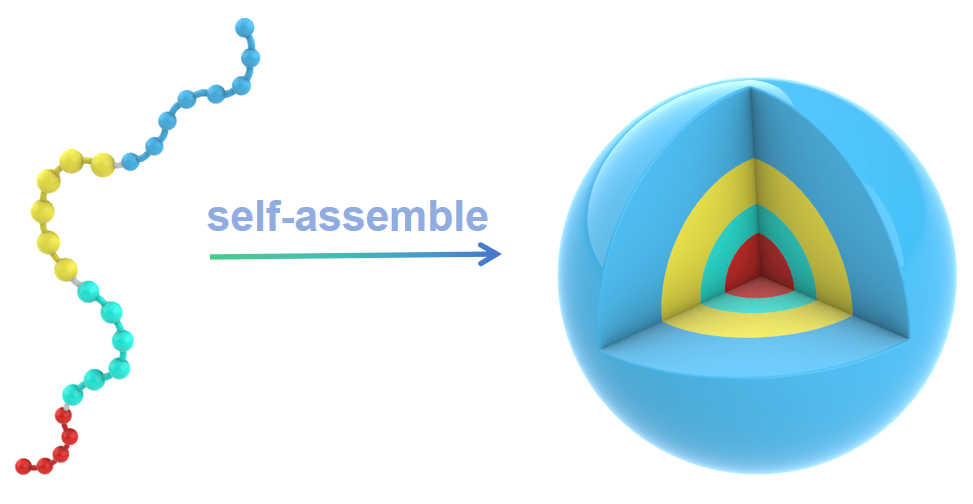
The peptide block copolymer has strong propensity to self-assemble into nanocapsules with a diameter of 100 nm in aqueous solution. The hydrophilic PEG-8 chain ensure the well-defined water solubility and biocompatibility of the peptide block copolymer.
1.EPR effect
The enhanced permeability and retention effect (EPR)[1] refer to the phenomenon by which molecules of certain sizes (typically liposomes, nanoparticles, and macromolecular drugs) tend to accumulate in tumor tissue much more than they do in normal tissue. The EPR effect rests on the fact that in solid tumors, the structural design of the vasculature is disorganized and abnormal (mostly characterized by higher vessel density), as compared with healthy tissue, and that lymphatic clearance from the tumor's interstitium is impaired[2]. Resulted from the EPR effect, our peptide-based nanocapsules with a diameter of approximately 100 nm tend to accumulate in tumor tissue much more better. This represents a form of passive targeting.
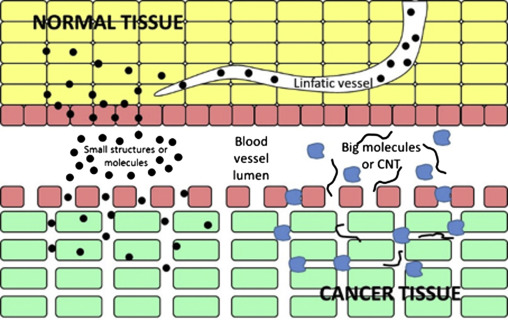
Diagram for the enhanced permeation and retention effect allowing to the nanoparticles or Carbon nanotubes(CNT) to be introduced into tumor tissue.
2.MMP-2-sensitive Peptide Linker
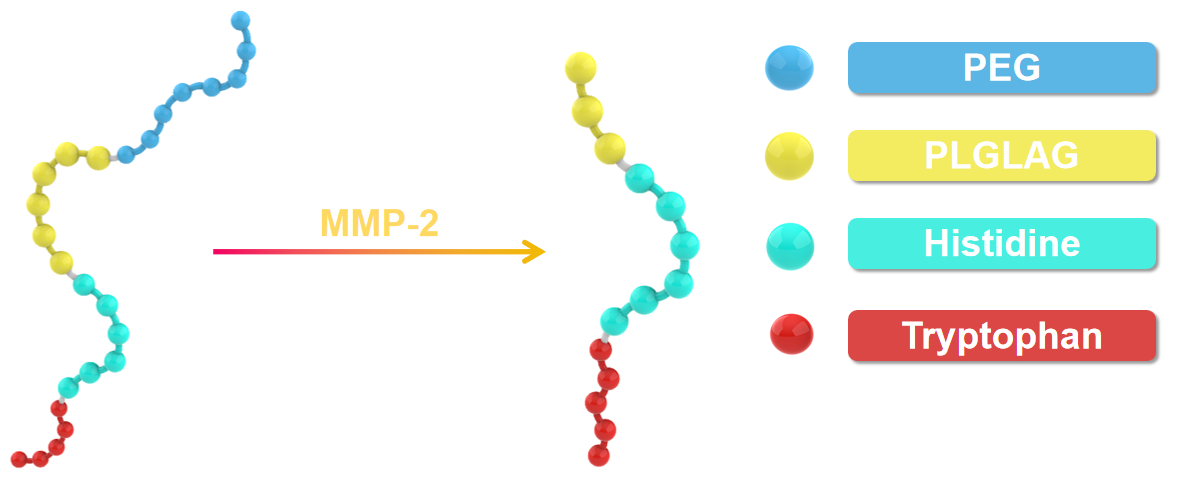
Matrix metalloproteinases (MMPs), particularly MMP-2, are recognized for their implication and over expression in various phases of human malignancies[3]. Currently, a range of MMP-responsive substrates have been developed and have demonstrated stimulus-responsive characteristics when employed in drug delivery and imaging systems[3]. As peptides with the PLGLAG sequence are sensitive to the MMP-2 being enriched in solid tumors[4,5], we use this sequence as a MMP-2-sensitive linker to conjugate pTrp-pHis unit to PEG-8. After accumulating in tumor tissue, the PLGAG linker can be cut off by the overexpressed MMPs, enabling the pTrp-pHis unit to be exposed in the acidic environment.
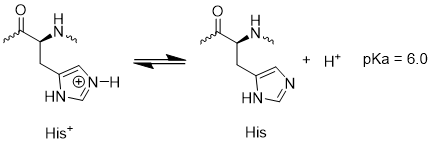
3.pH-responsive Polyhistidine Block
Typically, the acidity in tumor microenvironment is stronger than it in normal tissue, primarily attributed to the rapid metabolic activity of tumor cells[6]. Being an alkaline amino acid, histidine with a pKa close to the pH of the acidic tumor microenvironment exhibits pH-sensitive protonation and solubility characteristics. In a neutral environment, histidine is uncharged and manifests hydrophobic properties, which are of importance in driving the self-assembly of peptide block copolymer together with the poly(tryptophan) segment. However, in the acidic milieu of tumors, histidine protonates to become positively charged while transitioning into a hydrophilic state[7]. As a consequence, the positively charged pTrp-pHis unit can electrostatically bind on the negatively charged surface of the tumor cells and subsequently insert into the membrane with the assistance of the hydrophobic pTrp segment, leading to the collapse of the membrane of the tumor cells.
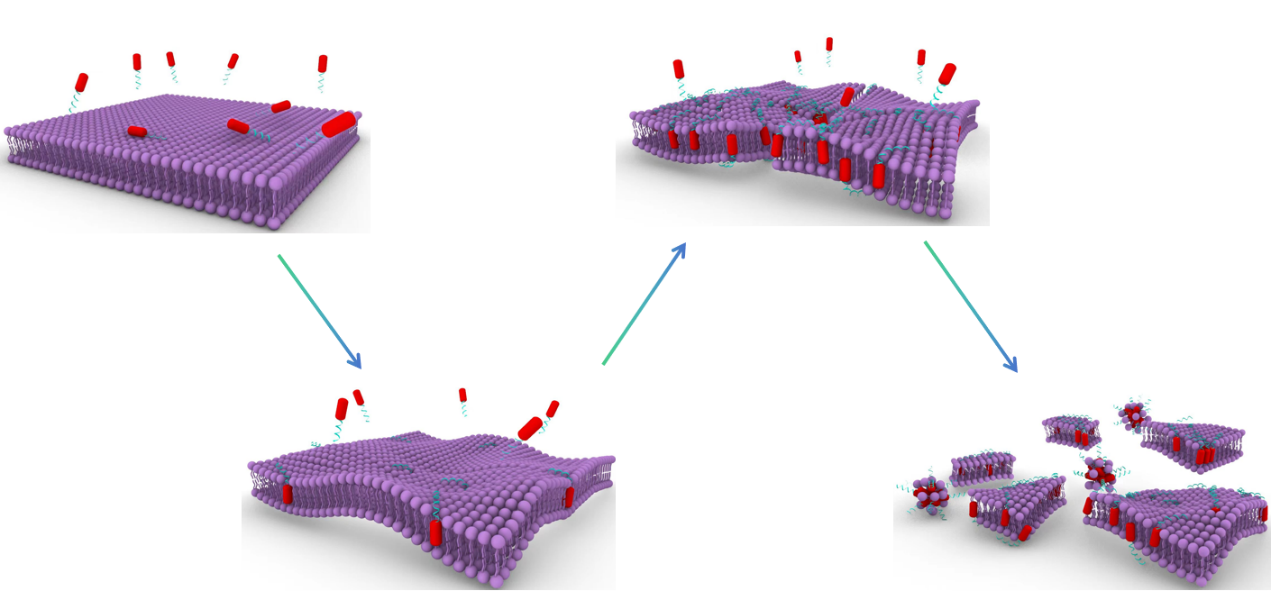
In conclusion, cleavage of the block-copolymer by MMP-2 and protonation of the pTrp-pHis block lead to collapse of the nanocapsules in a synergistic manner.
Plasma membrane rupture (PMR)-induced cell death typically bypasses the intracellular signaling pathways of target cells and ignores their drug-resistance spectrum and metabolic heterogeneity, presenting a promising approach for addressing drug-resistant pathogen infection and cancer[8]. In our design, after the collapse of the nanocapsules, the released cationic pTrp-pHis blocks engage in stable electrostatic interaction with the negatively charged tumor cell membrane, eventually inducing PMR-mediated tumor cell death. Prior research has indicated that the indole moiety of the tryptophan side chain exhibits notable PMR-toxicity[9].
[1]Nichols, J. W.; Bae, Y. H., EPR: Evidence and fallacy. J Control Release 2014, 190, 451-64.
[2]Alasvand, N.; Urbanska, A. M.; Rahmati, M.; Saeidifar, M.; Gungor-Ozkerim, P. S.; Sefat, F.; Rajadas, J.; Mozafari, M., Therapeutic Nanoparticles for Targeted Delivery of Anticancer Drugs. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics, 2017; pp 245-259.
[3]Zhu, L.; Kate, P.; Torchilin, V. P., Matrix Metalloprotease 2-Responsive Multifunctional Liposomal Nanocarrier for Enhanced Tumor Targeting. ACS Nano 2012, 6 (4), 3491-3498.
[4]Nagase, H.; Fields, G. B., Human matrix metalloproteinase specificity studies using collagen sequence-based synthetic peptides. Biopolymers 1996, 40 (4), 399-416.
[5]Dorresteijn, R.; Billecke, N.; Schwendy, M.; Pütz, S.; Bonn, M.; Parekh, S. H.; Klapper, M.; Müllen, K., Polylactide‐block‐Polypeptide‐block‐Polylactide Copolymer Nanoparticles with Tunable Cleavage and Controlled Drug Release. Advanced Functional Materials 2014, 24 (26), 4026-4033.
[6]Whiteside, T. L., The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27 (45), 5904-5912.
[7]Iwasaki, T.; Tokuda, Y.; Kotake, A.; Okada, H.; Takeda, S.; Kawano, T.; Nakayama, Y., Cellular uptake and in vivo distribution of polyhistidine peptides. J Control Release 2015, 210, 115-24.
[8]Liu, M.; Huang, L.; Zhang, W.; Wang, X.; Geng, Y.; Zhang, Y.; Wang, L.; Zhang, W.; Zhang, Y. J.; Xiao, S.; Bao, Y.; Xiong, M.; Wang, J., A transistor-like pH-sensitive nanodetergent for selective cancer therapy. Nat Nanotechnol 2022, 17 (5), 541-551.
[9]Chen, Y. F.; Lai, Y. D.; Chang, C. H.; Tsai, Y. C.; Tang, C. C.; Jan, J. S., Star-shaped polypeptides exhibit potent antibacterial activities. Nanoscale 2019, 11 (24), 11696-11708.